Chemical Treatments
Formic Acid
Lactic Acid
Oxalic Acid Safety
Oxalic Acid Properties
|
|
|
|---|
|
Chemical Treatments Formic Acid Lactic Acid Oxalic Acid Safety Oxalic Acid Properties |
Oxalic Acid Treatment
|
|---|
Oxalic acid may be used in both the liquid form, and as crystals that can be evaporated by electric heater pans, for treatment of honey bees with varroa infestation.... Each of the links in the table below, gives a link to the detailed method, as well as a brief synopsis of what it entails.
Spray method
Dilute oxalic acid is sprayed on to bees directly frame by frame.
Dribble method
A solution of oxalic acid, sucrose and water is dribbled along the seems of clustering bees during cold conditions that cause the bees to cluster.
Evaporation method
Solid crystals of oxalic acid are heated so that they evaporate. The resulting vapour treats the bees by fumigation.
|
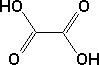
Oxalic acid, also called Ethanedioic Acid, as purchased is usually in the form of oxalic acid dihydrate, which is a crystalline form with two water molecules attached to each oxalic acid molecule. |
|
|---|
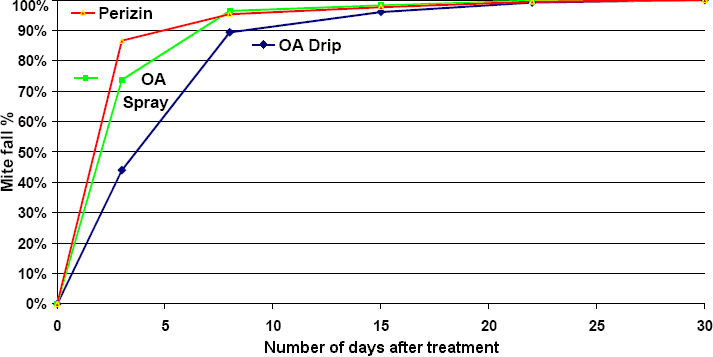
A comparison has been made of the delay after treatment of the mite fall with different winter treatments The study was carried out in year 2000 by someone named Wohlei, using Perizin as a control, reported by Jean-Daniel Charrière, who works at the Liebefeld research centre.
Basic properties of Oxalic acid...
Colourless, crystalline, water soluble, solid, toxic, organic compound with specific gravity of 1.6 - 1.7
If heated the water of hydration boils off at 101/102 °C leaving anhydrous oxalic acid crystals.
On further heating to between 149 and 160 °C, the oxalic acid sublimes from a solid to a gas)
At 189 °C the oxalic acid decomposes into formic acid and carbon monoxide.
|
It cannot be stressed too strongly that oxalic acid is an aggressive substance and needs to be treated with respect. Acid resistant gloves and goggles should be worn and an apron of the type used by mortuary attendants, along with wellington boots that have the tops covered by gaiters so that any falling liquid cannot fall into the boot. A respirator that has specialised organic acid filtering will be required in cases where the acid is sprayed or vapourised. Oxalic acid is also poisonous to humans by ingestion. |
Dave Cushman.
Page created 22/10/2002
Page updated 12/12/2022
Originated... 22 October 2002, Upgraded... 26 October 2005, Additions... 30, 31 October 2005,
|